Primary liver cancer (PLC) is the second most deadly malignant tumor worldwide, and incidence rates are rising, mainly owing to the increase in related risk factors such as diabetes or obesity. The majority of PLC cases are classified into hepatocellular carcinoma (HCC), cholangiocarcinoma (CC), and a combined hepatocellular-cholangiocarcinoma (CHC) subtype. PLC is characterized by genetic complexity and diversity, including high degree of aneuploidy, DNA copy number variations, somatic mutations, and epigenetic alterations that drive neoplastic transformation and growth. This renders each case of the disease unique and requires personalized treatment.
Historically, preclinical models are mainly composed of genetically engineered mouse models (GEMMs) or human tumor-derived cell lines propagated in 2D culture or as xenografts in mice. There are several limitations in using 2D culture: 1) limited number of established cell lines; 2) low efficiency of establishing new lines from primary tissues; 3) only aggressive tumors with high changes can establish in vitro culture successfully. Thus, the majority of primary tissue (>90%) cannot be successfully culture in vitro, especially the benign or less aggressive tumors. GEMMs provide insights into the biology of the disease, however, they are time-consuming, costly and cannot recapitulate all the human tumor features. As an alternative to GEMMs, patient-derived xenografts (PDXs) have been established, in which cells from patients (HCC and CC) are transplanted into immunocompromised mice. Although PDXs show great translational potential to direct treatment in a patient-tailored manner, this strategy has several disadvantages: PDXs are not amenable to large-scale drug screens, are costly, and may take a lot of time to establish.
The advent of the 3D culture system has made it possible to partially recapitulate the complexity and function of mammalian tissues in vitro, by producing structures similar to adult organs that have been termed "organoids". Organoid model system will provide huge opportunities to advance the research on liver cancer, drug development and personalized medicine.
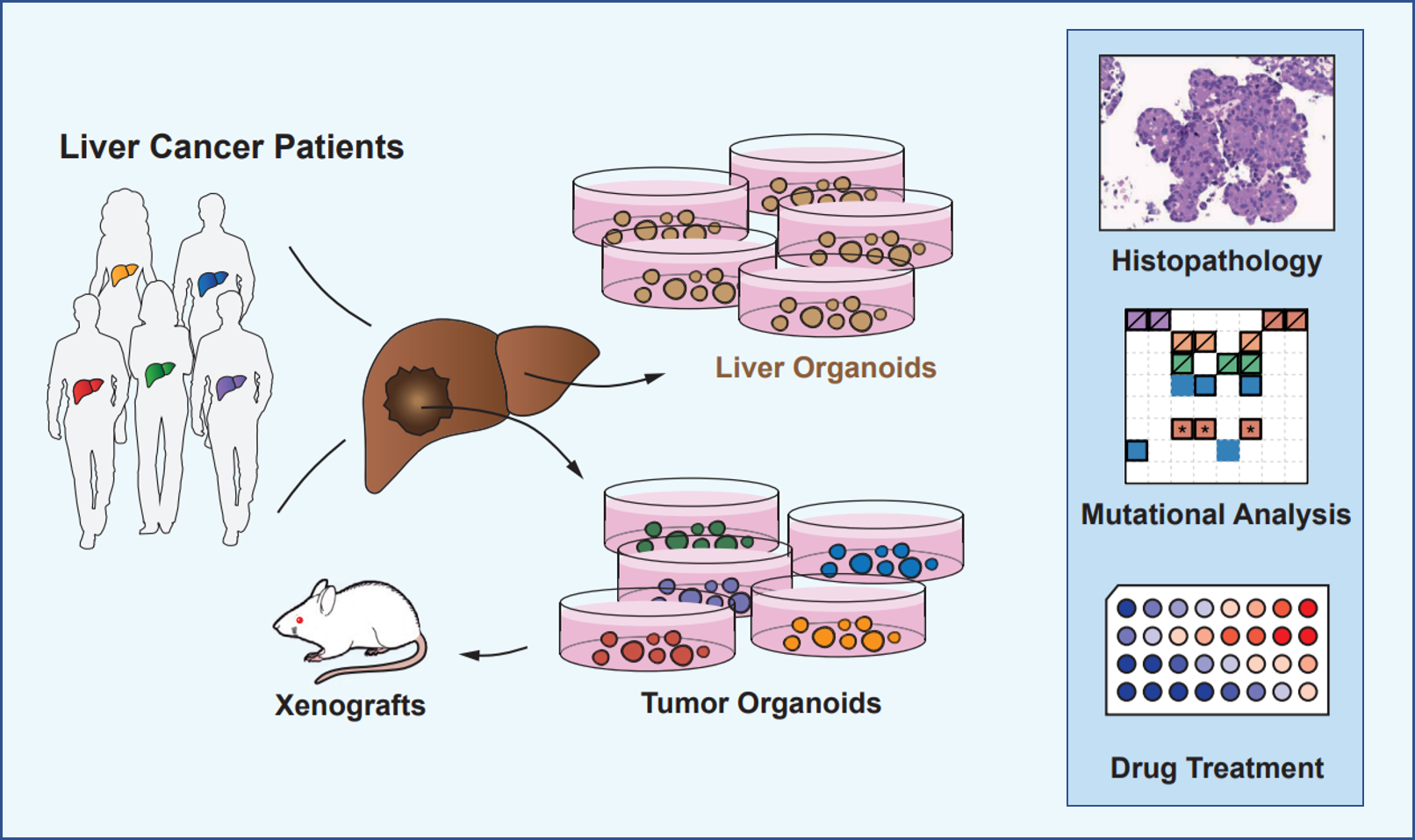
OrganoLab adopted protocols that established 3D organoids from primary liver cells and modified it to generate organoids from primary liver tumors. These organoids can be expanded in long-term cultures and initiate tumors in immunodeficient mice. Importantly, these organoids recapitulate the heterogeneity of liver cancer as seen in patients to a certain extent, with respect to phenotype, cancer cell composition and treatment response.
References
- Wu LJ. et al.; Organoids of liver diseases: From bench to bedside. World J Gastroenterol. 2019, 25(16): 1913-1927.
- van Tienderen GS. et al.; Recreating Tumour Complexity in a Dish: Organoid Models to Study Liver Cancer Cells and their Extracellular Environment. Cancers (Basel), 2019, 11(11): 1706.
- Tharehalli, U. et al.; Remodeling and Improvements in Organoid Technology to Study Liver Carcinogenesis in a Dish. Stem Cells International, 2019, 1-8.
- Cao W. et al.; Modeling liver cancer and therapy responsiveness using organoids derived from primary mouse liver tumors. Carcinogenesis, 2019, 40(1): 145-154.
- Broutier L. et al.; Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017, 23(12): 1424-1435.
- Nuciforo, S. et al.; Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Reports, 2018, 24(5): 1363-1376.
Online Inquiry
