Pancreatic ductal adenocarcinoma (PDA) is one of the most aggressive forms of cancer, and the third leading cause of cancer-related mortality in the United States. There are many reasons for this pathological outcome, including diagnosis at late stages, rapid progression with metastasis to other organs, and resistance to conventional therapeutic methods. Most patients are diagnosed with metastases and succumb to disease within 6-12 months of diagnosis. Therefore, understanding the mechanisms underlying disease initiation and progression are essential for early detection, risk stratification, and targeted therapeutic strategies.
In vitro and in vivo preclinical models that mimic the morphological and genomic characteristics of PDA have always been the focus of attention. The recent development of PDA organoids, which represent micro-scale mini-tumors, provides a promising new option for personalized drug testing based on materials from primary PDA patients.
Organoids are small tissue fragments extracted from larger organs and grown into 3 dimensional (3D) cultures, so they may eventually expand into ex vivo organ-like structures. Pancreatic cancer organoids are tumor models of individualized human PDA that can be quickly generated from surgically resected neoplasms. Organoids mimic the full spectrum of a patient’s tumor, including stromal elements believed to be responsible for chemotherapy resistance. Unlike patient-derived xenografts (PDXs) that require a relatively large amount of tumor tissue and may take several months to establish in the host organism, human PDA organoids can be generated rapidly and from a smaller amount of material. The organoids may then be used for personalized cancer treatment by testing potential therapeutic targets.
We have established organoid cultures from normal pancreas, PDA, and metastases. These cells proliferate in the presence of a defined set of growth factors and differentiation regulators that support the propagation of both normal and neoplastic cells within Matrigel, a 3D growth support matrix.
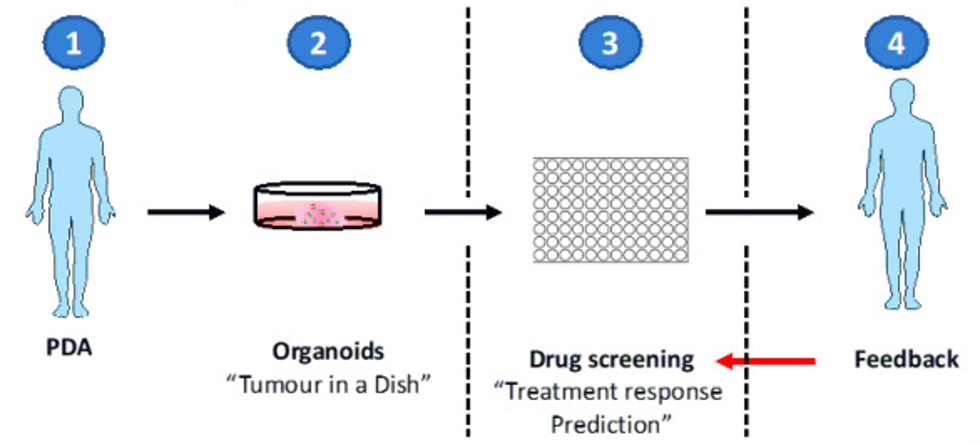 Figure 1. Organoids are isolated and expanded for drug screening.
Figure 1. Organoids are isolated and expanded for drug screening.
OrganoLab's organoid technology provides researchers with a "living" tissue biobank for discovering and validating new drugs and targets as well as modeling resistance to treatment. Using a set of patient samples from which screening can be done for drugs and mutations to understand why some patients respond to a particular treatment while others do not.
References
- Boj S. F. et al.; Model Organoids Provide New Research Opportunities for Ductal Pancreatic Cancer. Molecular & Cellular Oncology, 2016, 3:1.
- Moreira L. et al.; Pancreas 3D Organoids: Current and Future Aspects as a Research Platform for Personalized Medicine in Pancreatic Cancer. Cell Mol Gastroenterol Hepatol. 2017, 5(3): 289-298.
- Tsai, S. et al.; Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer, 2018, 18(1): 335-348.
- Tiriac, H. et al.; Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy for personalized cancer treatment. Gastrointestinal Endoscopy, 2018, 87(6): 1474-1480.
- Frappart, P.O. et al.; Pancreatic Ductal Adenocarcinoma (PDAC) Organoids: The Shining Light at the End of the Tunnel for Drug Response Prediction and Personalized Medicine. Cancers (Basel). 2020, 12(10): 2750.
- Baker L. A. et al.; Modeling pancreatic cancer with organoids. Trends Cancer. 2016, 2(4): 176-190.
- Nelson, S.R. et al.; Modelling of pancreatic cancer biology: transcriptomic signature for 3D PDX-derived organoids and primary cell line organoid development. Scientific Reports, 2020, 10(1): 2778.
Online Inquiry
