Bladder cancer (BC), referred to as urothelial carcinoma (UC), is the most frequent neoplasm of the urinary tract. BC is associated with high morbidity, mortality, and high costs for treatment. However, the absence of model systems to study the normal and malignant bladder epithelium (urothelium) hampers the development of new therapeutics.
Current strategies for disease characterization and therapy development rely heavily on two-dimensional (2D) cell culture technology and subsequent employment of animal models, which lead to high research costs. However, 2D cell culture models are insufficient to capture the complexity of in vivo architecture, such as extracellular matrix and cell-cell interactions that occur in 3D space. On the other hand, animal models are often unable to summarize the pathologies and treatment responses that occur in humans. Regenerative medicine provides novel mechanisms for characterizing pathologies and developing treatments for bladder diseases. One promising field involves the use of organoids, which are thought to closely recapitulate the in vivo environment. As a functional unit, organoids are composed of multiple cell types and contain multicellular organ structures, mimicking the tissue of origin and possessing multiple characteristics of the original tumor. Therefore, 3D organoid cultures derived from the tumor tissues improve our understanding of the disease biology. The 3D organoid cultures derived from BC patient tissue closely represent the original tissue genetically and histologically and are effectively used in molecular research and preclinical anticancer drug testing.
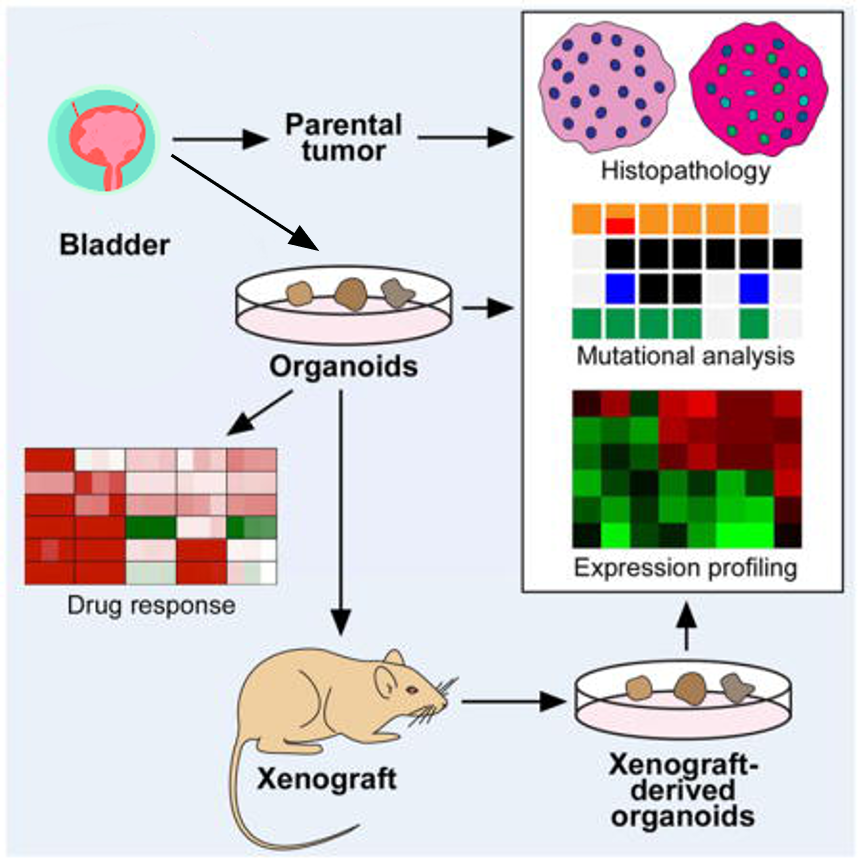
In OrganoLab, a biobank of patient-derived bladder cancer organoids that faithfully recapitulate the original tumor has been developed. Resulting organoids were characterized histologically and functionally. Organoid lines contained both basal and luminal bladder cancer subtypes based on immunohistochemistry and gene expression analysis. These BC organoids are convenient models for preclinical testing of potential therapeutic modalities, and can be serially passaged, cryopreserved, and xenografted.
References
- Whyard T. et al.; Organoid model of urothelial cancer: establishment and applications for bladder cancer research. Biotechniques, 2020, 69(3): 193-199.
- Jasper, M. et al.; Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proceedings of the National Academy of Sciences, 2019, 116(10): 4567-4574.
- Lee S. H. et al.; Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell, 2018, 173(2): 515-528.
- Stone, L. In the bank: bladder organoids recapitulate original tumour. Nat Rev Urol. 2018, 15(7): 397.
- Kim, Y. et al.; Culture, Manipulation, and Orthotopic Transplantation of Mouse Bladder Tumor Organoids. J. Vis. Exp. 2020, 155.
- Vasyutin I. et al.; Bladder Organoids and Spheroids: Potential Tools for Normal and Diseased Tissue Modelling. Anticancer Res. 2019, 39(3): 1105-1118.
Online Inquiry
